“Our goals are to support the in-vitro diagnostics industry, from start-ups, distributors to multi-national manufacturers, to understand, evaluate and implement quality and regulatory compliance, to allow placing safe and effective diagnostics onto the market, and ensuring patient and consumer safety.”
Stuart Angell & Nancy Consterdine
Co-founders
What Is An IVD?
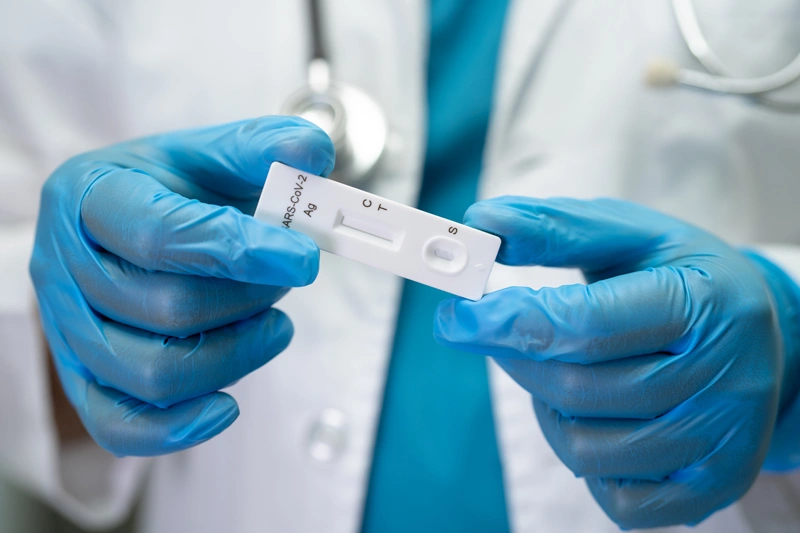
In-vitro diagnostics (IVDs) are a specific type of medical device, used to test human samples (e.g. blood urine, saliva). These tests can be used by a healthcare professional, or a lay user, to detect, or monitor a physical condition or infection. IVDs play an important role in the diagnosis of illnesses and support further medical treatment.
Due to the importance of the result from these tests, the design and development of these devices are highly regulated to ensure patient and user safety.
Our Services
Want to know more…?
Contact Us Directly
[email protected]
Monday-Friday 8.30 – 5.00pm only
@ivdeology-ltd