Last month the TGA, the competent authority in Australis has issued an update on the Transition to new manufacturer evidence for IVD medical devices (tga.gov.au). This document explains the new requirements for manufacturers to demonstrate that they meet the requirements for IVD in Australia.
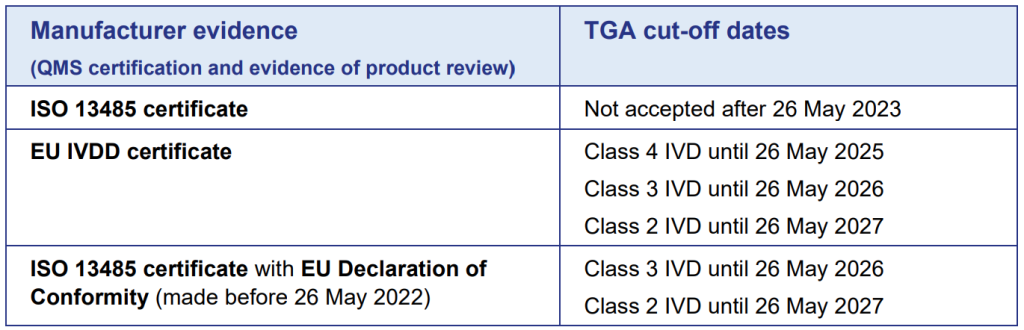
Typically, the use of CE marking has been used to demonstrate compliance to the TGA requirements, and the EU declaration of Conformity, along with an ISO 13485 certificate. However, with the transition to the IVDR, the TGA are requiring new evidence to be provided.
For new devices, an alternative to an ISO 13485 certificate is required, namely an MDSAP certificate covering ISO 13485 requirements. They will also be accepting IVDD certificates with existing ISO 13485 certificates during, and aligned to the IVDR transition dates.
Summary of TGA cut-off dates