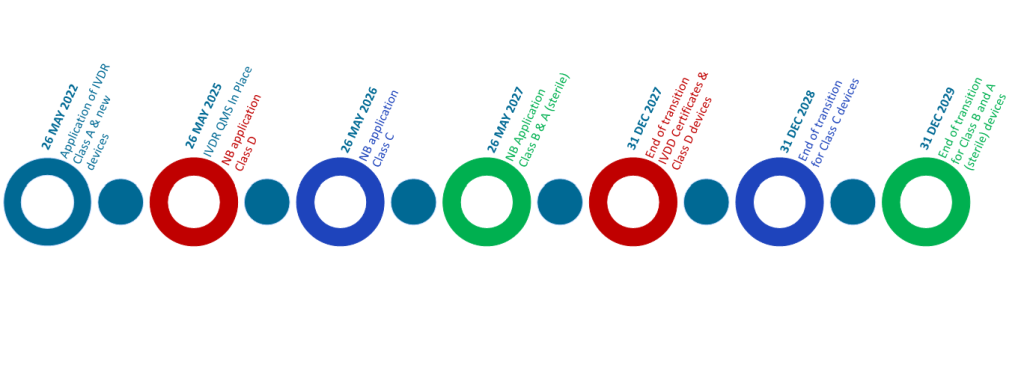
Before manufacturers can places in-vitro diagnostic medical devices (IVDs) on to the European Market for commercial use, they must meet the requirements of the European IVD Regulation – IVDR (2017/746/EU), and obtain a CE mark. The manufacturer must demonstrate that the product is safe, effective and meets the relevant regulatory requirements. This is done by preparing a series of technical documents.
What comprises Technical Documentation under the IVDR?
In Vitro Diagnostic (IVD) Technical Documentation is a crucial part of the regulatory process for IVD devices. It demonstrates conformity to the General Safety and Performance Requirements (GSPRs) according to the Annex I of the regulation. The documentation must reflect the current status of the IVD medical device through the application of the manufacturer’s Quality Management System (QMS). The GSPR covers 3 broad areas, described within 3 chapters:
- General requirements
- Requirements regarding performance, design and manufacture
- Requirements regarding information supplied with the device
The layout of the Technical Documentation required for the IVDR is much more defined compared to the previous approach under the IVD Directive. The manufacturer is required to compile the documents in line with the requirements of Annex II (technical documentation) & III (post market surveillance). The structure laid out in Annex II allows a more consistent approach for creating technical documents for IVDs, with sufficient ability to include or exclude (with sufficient justification) some elements depending on the specific nature or intended purpose of the device.
Once these have been met, the manufacturer can then draw up a Declaration of Conformity stating that the device is in compliance with the relevant regulation. For higher risk classes B-D, this will be done on completion of a Conformity Assessment process performed by an EU Notified Body.
What are the requirements?
The file shall include the following sections (these are the main headings in Annex II:
- Device Description and Specification
- Information Supplied by the Manufacturer
- Design and Manufacturing Information
- General Safety and Requirements (Annex I)
- Benefit-Risk Analysis and Risk Management
- Product Verification and Validation
The data above is required to be presented in a clear, organised, readily searchable and unambiguous manner, so that the documents can be easily read and understood. The aim of the technical documents are to provide a reader with a narrative of how the device has been designed and developed and is safe and effective when used as intended.
In most cases, the documents shall be presented to the notified body (for a class B, C and D device) electronically, so it would be advantageous to prepare and hold all documents electronically, ideally within a dedicated electronic Quality Management System (typically compliant to ISO 13485)
What data do you need to provide?
While some elements of the Technical Documentation is well defined, for example, you must provide all labels and instructions for use in the applicable languages of the European Union, much of the content can vary depending on the function, technology and intended purpose of the device, as long as the basic structure of the table of the contents remains consistent to Annex II.
When compiling your documents, it is well worth understanding any specific requirements based on how the device is used, e.g. if a self-test or near patient test, or with an integrated software element, as this will require additional information to be provided. It is also recommended to discuss your technical documentation layout with your notified body, who may have published specific guidance in how the documents should be presented. In addition to this, refer to guidance from Team AB (the European association of Medical Device Notified Bodies).
Final Thoughts
Both manufacturers and Notified Bodies have an interest to ensure a speedy technical review period. The closer Manufacturers align with the structure of Annex II, provide sufficient evidence to demonstrate conformity in a way that can be easily understood, the quicker (and cheaper) the review process will be.
It should also be remembered that the IVDR technical documentation acts as an iterative and traceable record telling the story of the history of the device. An effective, well-constructed technical file can be of huge benefit when retrospectively reviewing design changes, post market surveillance and risk management for your devices.
IVDeology is ideally placed to support IVD manufacturers in the completion of technical documentation to support CE submission. We have a successful and established process of building template structures, gap analysis and implementation. For any support required for CE marking, please contact us for a chat by clicking here
Written by Stuart Angell, MD and founder of IVDeology Ltd and IVDeology UKRP Ltd
#MDR #IVDR #diagnostics #technicalfile #techfile #invitrodiagnostic #cemark