If you are planning to place an in vitro diagnostic medical device (IVD) onto the market, It is critically important that you consider your regulatory strategy at the earliest opportunity. In our experience, building an effective strategy for regulatory strategy can be the difference between achieving product market access or not.
What is a Regulatory Strategy?
A Regulatory Strategy is a documented plan of all regulatory activities and deliverables that are required to be performed by a Legal Manufacturer. It should also align with the overall objectives of your organisation as defined within your vision, mission and business plan.
This should lay out a framework for placing an IVD on the market or markets depending on your commercial opportunity. Typically, the organisation will identify a priority list of countries where it is intending to sell the devices, the regulatory strategy describes the order of which these countries are being registered in.
Once the device is on the market, the plan continues to drive the regulatory surveillance mechanisms described as part of Post Market Surveillance as part of your ISO 13485 Quality Management System. It should also include a plan for meeting any transitions to updated regulatory requirements or ISO standards associated with the device.
Why do you need one?
For devices being placed on the European market under European IVD Regulation (IVDR), Article 10 (Manufacturers Obligations) describes the requirement for maintaining a regulatory strategy:
The quality management system shall address at least the following aspects:
(a) a strategy for regulatory compliance, including compliance with conformity assessment procedures and procedures for management of modifications to the devices covered by the system;
Furthermore, Annex IX requires the Quality Management System to include within its procedures, “a strategy for regulatory compliance, including processes for identification of relevant legal requirements, qualification, classification, handling of equivalence, choice of, and compliance with, conformity assessment procedures.”
In addition to the regulatory expectations, it is also hugely beneficial to plan and document the path to device registration and beyond. It also offers clear evidence to investors that the route to market has been considered and is planned.
When should you create one?
Typically, the regulatory strategy is formed, at a basic level, early on within the design and development process. Once your business vision and mission has been identified, and your business plan establishes the potential for the development of an IVD, the route to achieving that vision should now be considered.
Developing a regulatory strategy is an iterative process as many of the elements required will not have been considered or nailed down. This is entirely normal, but it is important to do the groundwork and start somewhere!
Our Approach to Regulatory Strategy
Education of the basic IVD requirements
A regulatory strategy can take many forms and will grow as you progress through the D&D process. We prefer to work with our SME customers and provide a regulatory strategy blended with some in-house training (virtually or on your site globally) on the key IMDRF principles, definitions and concepts of the regulation of IVDs. We aim to cover the main markets notably the EU, UK and MDSAP countries, to give you the best possible start in understanding what you need to know before developing your device.
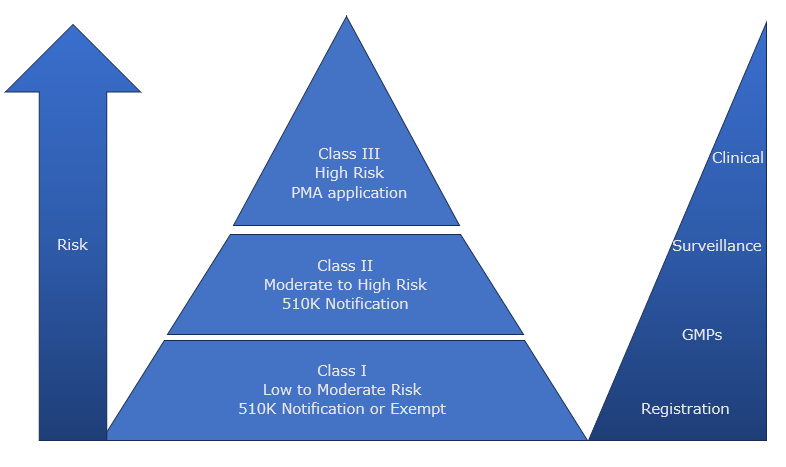
Evaluate device type and classification and routes to market
Using your existing ideas of what your device is, and how it should be used, we can help you construct an Intended Purpose Statement, which is the bedrock of how IVDs are classified and assessed. We will guide you through how to assign device nomenclature including GMDN or EMDN codes to understand the routes for conformity assessment and submission requirements, EU Notified Bodies or UK Approved bodies as required. The output of this evaluation will be detailed within a Regulatory Strategy Report, which you can share with your wider team and investors.
The strategy should sit alongside the Quality Plan, used to identify and plan the implementation of a Quality Management System (QMS).
End to end planning
We can incorporate our knowledge to help you understand an estimation of design and development stages with associated costs. This will help you identify when you need to grow and when to secure additional funding. Our experience however is that bringing an IVD to is never a straight line and your regulatory strategy may change over time. We can give you the background knowledge and tools along the way to navigate the complexities and challenges that you may face.
Be part of the journey with you
As part of the Abingdon Health group, IVDeology can spend time with you to understand, explain and build your regulatory strategy together that works towards your timelines and business project goals, but not only that, we can work together as a strong technical team with your business to support any gaps you may need. IVDeology building your regulatory plan with you means you always have a supportive hand for any questions, queries or concerns every step of the way with a team who knows your goals and vision.
If you’d like to discuss a regulatory plan, whether you have an existing one already or starting from scratch, you can book a call here, or email us on info@ivdeology.co.uk and we’d be happy to help!